Abstract
Background: Hemophilia A (HA) is a bleeding disorder characterized by decreased or absent FVIII. Clinical analysis of coagulation potential in this patient population is classically based on APTT based FVIII assays. Although both the one-stage FVIII assay and the chromogenic FVIII assay can measure FVIII concentrations reliably these types of assays only give insight on the initiation of coagulation. Global coagulation assays, like thrombin generation (TG), can be used to measure the full coagulation spectrum of initiation, amplification and propagation. However the frequently used commercially available TG kits lack sensitivity for measurements of hemophilia plasma within the lower FVIII ranges which are essential in explaining differences in bleeding phenotype.
Aim: We aim to optimize the sensitivity of the TG-assay for measurements in hemophilia A patients, especially in the lower FVIII ranges.
Methods: In order to minimize patient specific sensitivity a hemophilia A pool plasma (HAPP) was created. Analysis of the influence of pre-analytical variables, like contact activation inhibitors, on the assay was performed. Initiation of coagulation by different reagents was compared for sensitivity towards factor FVIII titrations in patient plasma. Other assay variables like phospholipids and temperature were adjusted to increase sensitivity even further.
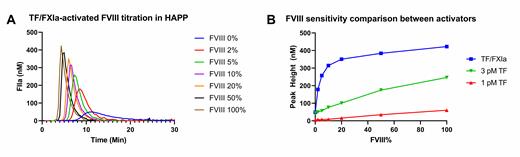
Results: Commonly used tissue factor (TF) initiated TG at varying concentrations was unable to significantly differentiate in FVIII levels below 20%. In contrast, TG activation with low concentrations of TF in presence of FXIa appeared to be highly sensitive for FVIII changes both in high and low ranges. Additionally, a representative baseline TG-curve in severe HA plasma could only be produced using this dual TF/FXIa-activation. There was a value in the addition of contact activation inhibitors in the assay. Higher phospholipid concentrations seem to benefit this assay setup compared to a TF only setup.
Conclusion: TF/FXIa dual activation thrombin generation increased assay sensitivity in severe hemophilia plasma, allows for dose-dependent measurements in low FVIII ranges and provides a solid baseline curve that can be used for further clinical evaluation of coagulation potential and possibly therapeutic monitoring in hemophilia A.
Hackeng: ACS Biomarker BV: Current Employment, Current equity holder in publicly-traded company; Coagulation Profile BV: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal